Innovation Acta News
2025
-
Oct9-10
B-specific 2nd Annual Meeting
We are glad to share that the 2nd Annual Meeting of B-specific took place at the Grand Hotel Lund, Sweden, between the 9th and 10th of October 2025. The meeting was organized by Lund University with support from Innovation Acta.
The meeting was a valuable opportunity for in-person discussions, sharing results, and planning future activities. An important part of the event was the roundtable discussion to assess results, upcoming tasks, risks, and mitigation strategies in R&D work packages.
Thank you to all partners for their commitment and contributions, and to the organizer for making the meeting possible and welcoming the B-specific consortium to Lund.

-
Oct1-3
T-FITNESS 3rd annual meeting
The 3rd Annual Meeting of the T-FITNESS consortium was successfully held in the beautiful city of Nice from October 1st to 3rd.
The event provided a valuable platform for researchers and partners to present recent advancements, review project progress, and align future activities. It emphasized the strength of our collaborative efforts and the importance of international partnerships in driving scientific innovation.

-
Sep10-12
INSIDE: INSIGHT Doctoral Candidates Kick Off and Workshop 1 Event
From 10-12 September 2025, The University of Pisa through Prof. Vincenzo Ferrari, and ENDOCAS Center, hosted the official PhD fellows' kick-off of INSIDE: INSIGHT, MSCA funded EU Doctoral Network (Grant Agreement 101168715) dedicated to integrating XR technologies into medical education. The event was full of inspiration, vibrant energy and exciting collaborations across Europe, Canada, and South Africa to explore the future of immersive learning in healthcare.

-
Jul21-22
INN-FORMA retreat at Certosa di Maggiano
Earlier this summer, our team gathered for the Innovation Acta “INN-FORMA” retreat - a wonderful opportunity to recharge, connect, and align our vision for the months ahead. The inspiration we carried from this experience has guided us through a very restorative and exciting season with a Pilates instructor from President Gym and kindly hosted by the marvelous scenery of Certosa di Maggiano!

-
May-Jun
Corso proposal writing Rome e IZSLER e Policlinico
Successful Proposal Writing Courses held in Milan, Rome, and Brescia
Over the past few weeks, a series of hands-on Proposal Writing courses organised and held by Innovation Acta Grant Managers took place at Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico in Milan (5-6 May 2025), Centro Congressi in Rome (5-6 June 2025), and Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emila Romagna - IZSLER in Brescia (18-19 June 2025), attracting researchers and professionals from across the country. The sessions focused on best practices for preparing competitive proposals under Horizon Europe programmes, offering practical guidance and the best strategies on structure, evaluation criteria, and impact planning. Participants engaged in interactive exercises, shared experiences, and received expert feedback on draft proposals. The strong turnout and active participation in all three cities reflect a growing interest in European funding opportunities and a commitment to excellence in research and innovation. More workshops are planned in the coming months — stay tuned!

-
Jun19-20
BIO2 Kick off meeting
The kick-off meeting of BIO2 project, coordinated by Alessio Adamiano (Consiglio Nazionale delle Ricerche (CNR)) was hosted at the CNR in Bologna, Italy, between 19th-20th June 2025. The BIO2 project entitled: Natural bio-mass and microbial bio-control agent solutions for the replacement of contentious inputs in organic farming (acronym BIO2), was funded under the call HORIZON-CL6-2024-FARM2FORK-02-1-two-stage, started on the 1st May 2025 and will last for 48 months. BIO2 consortium is composed of 20 partners from 8 countries (Italy, Norway, Latvia, Spain, Bulgaria, Germany, Sweden, Austria), including 6 industrial partners, of which 2 are SMEs. The 2-day meeting started with the welcome of the Coordinator and Director of the CNR - Institute of Science, Technology and Sustainability for Ceramics (CNR-ISSMC). During the meeting, each beneficiary presented their role and expertise, while Work Package (WP) leads discussed activities, upcoming tasks, deliverables, milestones, and risks, emphasising collaboration among beneficiaries and interaction with other WPs. Project and Policy officers from the European Research Executive Agency (REA) provided valuable insights on project management, reporting, dissemination, communication, and policy support. Innovation Acta co-organised the event with CNR and plays a role in supporting communication, event organisation activities, and assisting with administrative issues.
We thank the European Commission for supporting the research.

-
Jun16-19
Corso CNR Napoli
Nell’ambito dei corsi organizzati da Innovation Acta, dal 16 al 19 giugno 2025 si è svolto a Napoli presso il Renaissance Naples Hotel Mediterraneo il corso di formazione per il Progetto H2IOSC - Humanities and cultural Heritage Italian Open Science Cloud finanziato dall’Unione europea NextGenerationEU - Piano Nazionale di Ripresa e Resilienza (PNRR) - Missione 4 “Istruzione e Ricerca”. Soggetto attuatore CNR.
Questi i temi trattati durante le quattro giornate:
Open Science, Open Access, FAIR Data, OPERAS (Elena Giglia), Diritto d’Autore (Simone Aliprandi), Oggetto Digitale (Alfredo Corrao), TEI (Marco Scarbaci), Rappresentazione dell’informazione e Ontologie per i Beni Culturali (Margherita Porena), Metadati e Gestione di archivi digitali (Giovanna Garofalo), Digital Library e I.PaC (Margherita Bartoli).

-
May27-28
IMMUTOL 2nd Annual Meeting
The 2nd Annual Meeting of IMMUTOL, organised by INN-Acta and hosted by Syreon Research Institute (SRI) took place in Budapest between the 27th and 28th of May 2025. The first day of the event included progress updates from young scientists on WP2 (optimizing monocyte collection), WP3 (developing advanced VitD3DC cells), and WP4 (pre-clinical safety and potency studies). This was followed by an in-depth brainstorming session with Advisory Board members on future R&D activities. The second day was focused on activities for WP7 (economic viability), WP8 (communication and engagement), and WP1 (project management), where INN-Acta contributed to WP1 and presented WP8. In addition, on the last day of the meeting, there was a two-hour seminar on regulatory guidance for engineered VitD3DC for human therapy, led by Asphalion (ASP) representatives. Representative of the European Multiple Sclerosis Platform (EMSP) also attended the meeting and provided a very important insight into EMSP activities and collaboration with IMMUTOL.

-
May28-29
SECRET Kick off meeting
SECRET Project Doctoral Candidates Kick off Meeting
The EU-funded project SECRET – Exploring the Therapeutic Potential of Perinatal Cell SECRETomes officially launched on May 28–29, 2025, with a Doctoral Candidates Kick-off Meeting at the Centro di Ricerca Eugenia Menni (CREM), part of Fondazione Poliambulanza in Brescia, Italy. Coordinated by Professor Ornella Parolini of Università Cattolica del Sacro Cuore (Rome, Italy), SECRET is a Marie Skłodowska-Curie Actions Doctoral Network under the Horizon Europe programme. The project aims to investigate perinatal secretomes—biological substances with regenerative potential—for treating neurological and cardiovascular conditions. The two-day event brought together representatives from 11 institutions across 6 EU countries, creating an atmosphere of collaboration and excitement. PhD fellows showcased strong engagement and drive, setting a promising tone for the project's future.
Click for more

-
May11-13
TOLERATE 2nd Progress Meeting
From May 11–13, the TOLERATE consortium gathered in Dublin for our 2nd Progress Meeting, hosted by Royal College of Surgeons in Ireland (RCSI). A special highlight was the active participation of our Doctoral Candidates, who presented their research progress and engaged in fruitful scientific discussions. In parallel, the meeting featured the Workshop on “Communicating with your stakeholders”, and a training module on clinical research entitled “The impact and role of standardized databases and biomarker testing for long-term follow-up clinical studies of iTTP patients” equipping our early-stage researchers with essential competencies beyond the lab.

-
May5-6
GUIDE.MRD 3rd General Assembly
The Innovative Health Initiative (IHI) GUIDE.MRD consortium partners met for the 3rd annual general assembly from 05-06 May 2025 in Nice, France. After a warm welcome to the beautiful city of Nice by the Academic Coordinator Klaus Pantel (UKE) and the Project Leader Bristol Myers Squibb (BMS) represented by Trixia Camacho and Luigi Ravagnan, as well as Paul Hofman (CHU Nice, RespirERA) the progress on all work packages was presented by the work package (WP) leads and co-leads. Moreover, a dedicated Patient Advisory Board (PAB) Session with Merel Hennink and Stephen Rowley representing the entire GUIDE.MRD PAB was held. Almost 50 participants from the partners engaged in very productive discussions on the results reached by the GUIDE.MRD consortium. The external GUIDE.MRD Scientific and Ethical Advisory Board (Iryna Lishchuk, Naureen Starling, Nitzan Rosenfeld, Yuval Dor) gave valuable feedback on the project.

-
May3-6
TwistedNano 3rd Annual Meeting
The TwistedNano project held its Annual Meeting with representatives from all of our partners. The meeting was hosted by our partner 9, University of Cyprus (Nicosia, Cyprus). After a warm welcome by the Sotirios Christodoulou (University of Cyprus) the progresses on all work packages (WPs) were presented by the WP leads and co-leads. The main objective of the meeting was to facilitate discussions within the TwistedNano Project Consortium on key advances and innovative approaches for the project. More importantly, the meeting aimed to address any challenges that arose and to propose solutions for the smooth progression of the project.

-
May20
Get Ready for the Upcoming Horizon Europe Health Calls!
The next Horizon Europe calls are expected to be published in the coming weeks — and it's time to prepare!
More

-
May20
Horizon Europe Info Days Coming Soon – Save the Date!
Are you planning to apply for EU research and innovation funding?
Don't miss the upcoming Horizon Europe Info Days, taking place in the next few days!
The Info Days offer a unique opportunity for prospective applicants and stakeholders to:- ✔ Get detailed information on upcoming funding opportunities
- ✔ Understand the application and evaluation processes
- ✔ Ask questions directly to the European Commission experts
Find more information here: Horizon Europe Info Days – European Commission
Mark your calendar and stay tuned for the full programme!

-
Apr07-09
Flash News: Results of HORIZON-MSCA-2024-DN-01 Call for Proposals
The evaluation of proposals for the HORIZON-MSCA-2024-DN-01 2024 call for Marie Skłodowska-Curie Doctoral Networks has been completed. A total of 1,417 proposals were submitted, with 12 found to be ineligible, inadmissible, or withdrawn. Out of the remaining, 1,237 proposals passed the evaluation threshold. The total funding requested for these above-threshold proposals amounts to €4.96 billion. The available budget for the call is €608.6 million. Applicants have been notified of their evaluation results. It is expected that the first grant agreements will be signed by July 27, 2025.
-
Apr07-11
Glyco-N workshop and mid-term meeting
As part of our ongoing efforts to foster collaboration and support the development of our doctoral candidates, the two-day workshop Structural N-Glycoscience: chemical/biophysical approaches toward understanding “novel N-glycans out of the blue” was successfully organized by Prof Antonio Molinaro at the University of Naples, bringing together valuable experts in the field and all enrolled doctoral students. The workshop provided a valuable opportunity for training, exchange of ideas, and team-building among the participants. In addition, a mid-term meeting was held with the presence of the REA Project Officer Flavia Buiarelli. During this important milestone event, each doctoral student had the opportunity to introduce themselves and present their research projects. The presentations were marked by a high level of enthusiasm and engagement, showcasing the diversity and quality of the work being undertaken within the project. These events reflect our commitment to creating a vibrant and supportive research environment, and we look forward to the continued progress of our doctoral researchers.

-
Apr9
Glyco-N mid-term meeting
-
Apr
TOP-GUT Training Event & Mid-Term Meeting with REA
Hosted by i3S - Instituto de Investigação e Inovação em Saúde, in the beautiful city of Porto, brought together TOP-GUT "family" for this unique learning experience. The week included a comprehensive training programme for the 11 talented doctoral candidates, that combined theoretical insights with hands-on practical sessions. A special highlight was the Annual Consortium Meeting, a fantastic opportunity for DCs to present their posters and share their preliminary results to the network, to the project officer and experts in the field who gave invaluable insights and feedback to further strengthen the work and the network.


-
Mar24-25
Highlights from the PROTO Second Annual Meeting in Siena
It was a fantastic opportunity for Innovation Acta to organize and host the PROTO Second Annual meeting on March 24-25, 2025 in the enchanting and historic city of Siena. PROTO partners shared insights, and discussed the progress made across all scientific work packages. We also had a chance to engage in communication strategies, with a special feedback session from patient representatives. Their insights are key to ensuring that our efforts align with patient needs. The meeting was complemented by an insightful guided tour of the beautiful city of Siena and a visit to the “Contrada dell’Onda” Museum to learn about the history and tradition of the Palio – a fantastic cultural experience! A huge thanks to all participants and speakers for making this event a success!

-
Mar20

CARB-X has opened their funding round for 2025!
The funding solicitation has two distinct product themes:
- THERAPEUTICS FOR INFECTIONS CAUSED BY GRAM-NEGATIVE PATHOGENS
The scope is restricted to direct-acting small-molecule therapeutics. Strategies requiring potentiator molecules (including, but not limited to, BLIs, efflux inhibitors, membrane permeators) are not within scope. Molecules with properties that will deliver an IV route with an oral stepdown are preferred. In all cases, activity against both susceptible and multidrug-resistant organisms on priority bacterial threat lists is essential. - DIAGNOSTICS FOR TYPHOID FEVER FOR LOW-RESOURCE SETTINGS
We seek diagnostics to support the portfolio to diagnose acute infection of Salmonella enterica serovar Typhi, the causative pathogen in typhoid fever. The primary health care level is the preferred use setting, with ease-of-use, high performance and affordability prioritized.
Target product profiles and minimal entry criteria for each theme will be defined and available on CARB-X.org. Expressions of interest may be submitted from 16 April 2025 at 10:00 ET – 30 April 2025 at 23:59 ET. Two public webinars will be held during the week of 14 April 2025 to discuss the scope of the funding round, application process, and to answer questions. Register for the CARB-X newsletter to receive updates.
CARB-X key features
- THERAPEUTICS FOR INFECTIONS CAUSED BY GRAM-NEGATIVE PATHOGENS
-
Feb17-18
Kick-off meeting of AUREUS
The AUREUS Doctoral Network Kick-Off Meeting was held at the Department of Chemical Sciences in Napoli, Italy, on February 17-18, 2025, with participation from the Project Officer of the European Research Executive Agency and an Advisory Board representative. The KoM was an excellent opportunity to discuss the scientific background of the project and get prepared for the recruitment of 15 Doctoral candidates. INN-ACTA, as Associated Partner in the consortium, will be involved in the organization of training courses, providing support for the organization of project meetings and communication and dissemination activities.

-
Feb11-12
EXTRA Consortium Mid-Term Review & Annual Meeting
We are delighted to have attended the EXTRA second annual and mid-term review meeting in Regensburg. The two-day event was filled with insightful sessions, and presentations by 11 exTra brilliant doctoral candidates—the driving force of this project—who showcased their experiments and key results from the first year of lab work. The event hosted external experts who provided valuable insights and shared achievements in the ECP field, enriching the discussions and inspiring future directions.

-
Feb6
THRIVE first annual meeting
The first annual meeting of THRIVE was held online on February 6, 2025, bringing together all Consortium partners, and members of the External Advisory Board. The meeting provided an opportunity to review the project's first-year achievements and discuss plans for the future. Innovation Acta facilitated the organization of the meeting and presented updates on project management, as well as dissemination and communication activities.
-
Feb4
InFlaMe project Kick-Off Meeting
The InFlaMe consortium, led by Professor Fausto Baldanti from the Fondazione IRCCS Policlinico San Matteo in Pavia, Italy, successfully launched a project entitled "Counteracting the Pandemic Potential of Flaviviruses: Addressing Virus-Host Interactions and Defense Strategies to Design New Therapeutics Against WNV and DENV" (InFlaMe), Project Number 101191725. The kick-off meeting took place at the Palazzo Bellisomi-Vistarino in Pavia on 4-5 February 2025. InFlaMe began on January 1, 2025, and will last for 48 months. The consortium consists of 11 partners from five countries: Italy, France, Austria, Spain, and Czechia. Innovation Acta is leading Work Package 7 (WP7), which focuses on communication, dissemination, exploitation, and a One Health approach to pandemic preparedness, ensuring impact beyond the project's lifetime. We thank the European Commission for supporting the research.


-
Jan31
THRIVE First Cluster – Tumor-Host Interactions annual meeting
The first annual meeting of the Understanding Cluster – Tumor-Host Interactions was succesfully organised in Barcelona on January 31, 2025, hosted by THRIVE and IDIBAPS. Innovation Acta played a key role in organizing the meeting and actively contributing to discussions on the key topics covered by the meeting. This milestone event marked a key step in our collective mission to unravel the complexities of tumor-host interactions and drive innovation in cancer research. Representatives from all six projects within the Cluster (THRIVE, MULTIR, GLIOMATCH, ARTURO, SPACETIME) and the HaDEA Project Officer Marianne Da Silva came together for insightful discussions on critical topics, including:
- Data Management and Sharing
- Communication and Dissemination
- Research and Innovation
- Addressing Inequalities
- Citizen and Patient Engagement
Through inspiring keynotes, engaging panel discussions, and collaborative networking sessions, we explored new opportunities to accelerate progress and maximize the impact of our research. We look forward to building on this momentum and continuing our journey toward meaningful scientific advancements.

-
Jan16-17
Successful Kick-Off Meeting of the BactEradiX Project
The Kick-Off Meeting of the BactEradiX Project took place in Bologna on January 16-17, marking a highly productive and goal-oriented start to this ambitious initiative. The event, organised by Innovation Acta, brought together all project partners, fostering valuable discussions and strategic planning. We were honored to have the participation of Project Officer Barbara Gerratana, who joined us online to share her insights and provide essential guidance for the project’s next steps. Funded by the European Innovation Council (EIC), BactEradiX represents a significant advancement in the fight against antimicrobial resistance, a growing global health challenge.

-
Jan24-25
NECESSITY Sixth General Assembly
The NECESSITY project held its annual General Assembly with representatives from all of our 25 partners, including both from industry and academia. We were also honored to have the indispensable Patient Advisory Group (PAG) representatives among us. The two-day meeting was hosted by our industry partner, BRISTOL MYERS SQUIBB - BMS in Boudry (Neuchâtel, Switzerland). The main objectives of the two days were to facilitate discussions within the NECESSITY Project Consortium on key advances and innovative approaches in the fields of immunology and rheumatology. More importantly, the meeting aimed to address any challenges that arose and to propose solutions for the smooth progression of the project. Exciting new methodologies and results were presented for the stratification of patients based on tissue and blood biomarkers. Additionally, we delved into an emerging approach in computational biology to evaluate the pathophysiology of Sjogren’s disease.

-
Jan8-9
GLYCO-N first annual meeting
The 1st Annual Meeting of the Glyco-N project was successfully held in Leiden, hosted by Universiteit Leiden on January 8th and 9th, and organised with the support of Innovation Acta. This event brought together all PhD candidates and supervisors, providing a platform to connect, share their backgrounds, and outline the project’s objectives. It was a fantastic opportunity to align our goals and lay the groundwork for future collaborations.

-
Jan
Other news
-
Jan
Other news
ReSHAPE video
We are thrilled to announce the launch of the 2D animated, multilingual video, created to engage and educate the general public— including patients and school children— about ReSHAPE project core mission and objectives. The video also highlights the crucial concept of immune balance and its essential role in promoting and maintaining overall health. Watch the video Understanding ReSHAPE Project and Immune Balance - Educating and Empowering Communities
-
Jan
Other news
Eurostar call for proposal is now open- Deadline 13 March 2025
Eurostars is a funding instrument that supports innovative SMEs and project partners (large companies, universities, research organisations and other types of organisations) by funding international collaborative R&D and innovation projects. By participating, organisations can access public funding for international collaborative R&D projects in all fields. More
-
Jan1
Upcoming calls 2025
IHI calls 9 and 10 - The final versions of the call texts are now available
On 11 December 2024, the IHI Governing Board adopted the IHI Work Programme for 2025. This includes the call texts for calls 9 and 10, which will be launched in mid-January 2025. Links to the relevant pages of the Funding and Tenders Portal will be published when the calls are officially launched.
IHI call 9 - a single-stage, applicant driven call
IHI call 9 will be a single-stage, applicant-driven call with five topics aligned with the five specific objectives set out in the IHI Strategic Research and Innovation Agenda (SRIA). Draft of IHI call 9
IHI call 10 - a standard, two-stage call
IHI call 10 will be a standard, two-stage call for proposals with the following topics
- Digital label: one source of comprehensive information for medical technology products
- Enabling and safeguarding innovation in secondary use of health data in the European Health Data Space (EHDS)
- Per- and Poly-fluoroalkyl substance (PFAS) exposure, emissions, and end of life management in the healthcare sector
New! Download the final, approved call 10 text
-
Jan1
Upcoming calls 2025
EPPerMed JTC 2025 "“Pharmacogenomic Strategies for Personalised Medicine”
Support research projects in human health on pharmacogenomic strategies for personalised medicine approaches that address one or more of the following aspects: identification of new pharmacogenomic markers or signatures using (multi)-omics data in relation to drug or drug combination; validation of a pharmacogenomic marker or signatures using (multi)-omics data in predicting drug or drug combination outcomes; use pharmaco-omics strategies to determine the right dosage, the efficacy of treatments and/or the risk of adverse drug response and non-response to treatment to tailor personalised treatment pathways, including combined treatments (multi-medication).
The call will open soon
18 February 2025, 14:00 CET -> Deadline for submission of pre-proposal
17 July 2025, 14:00 CEST -> Deadline for submission of full-proposals
Text of the call
-
Jan1
Upcoming calls 2025
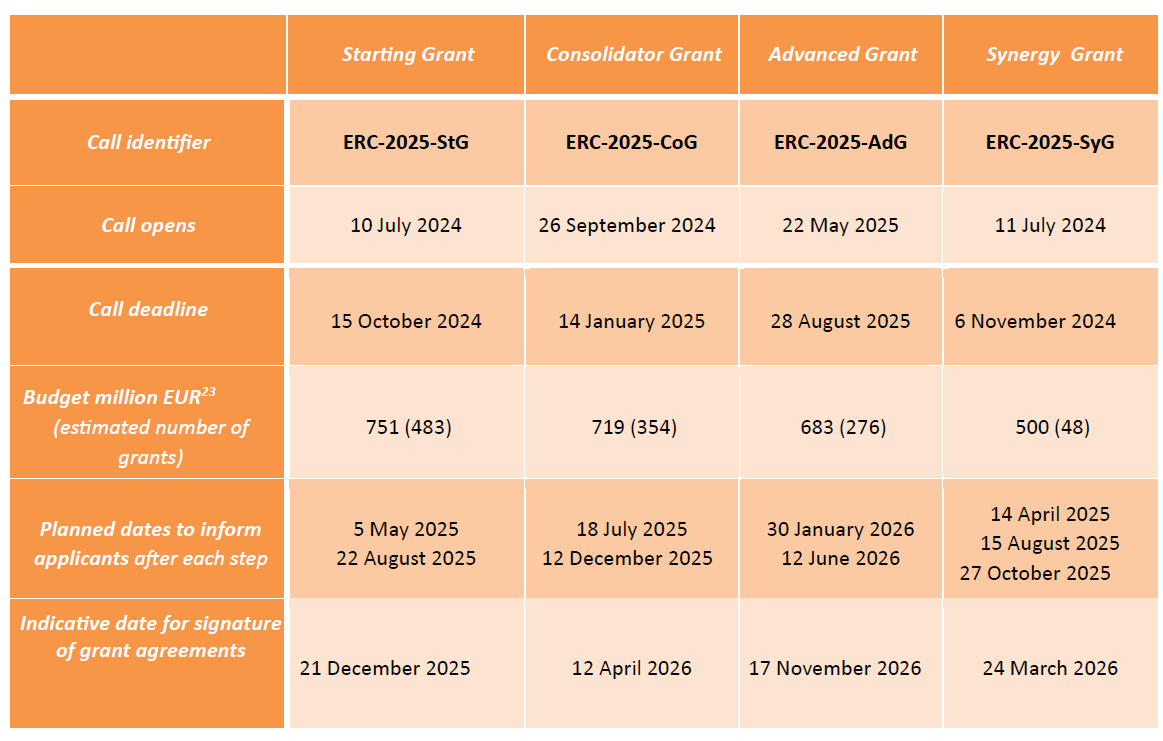
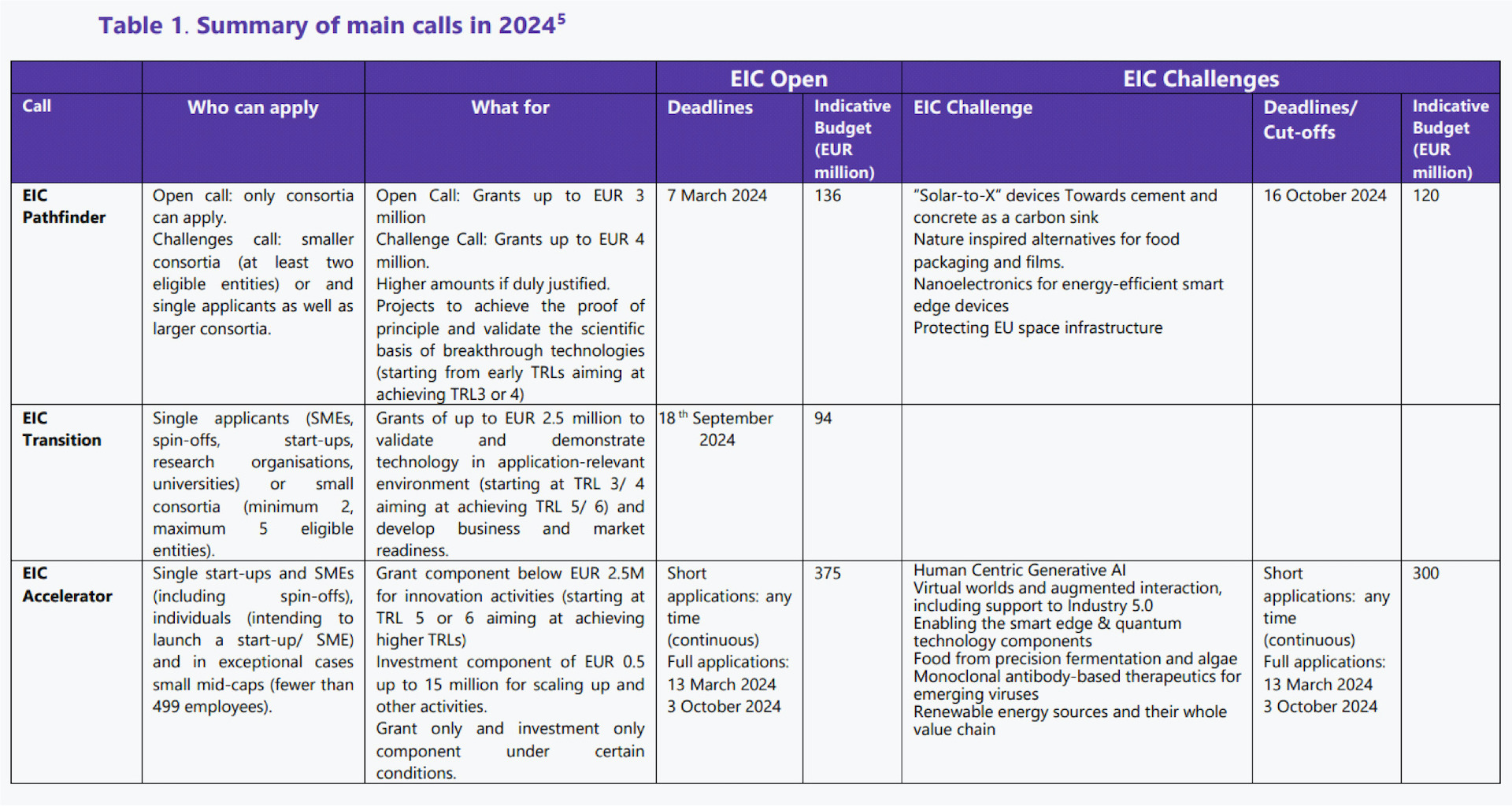
The EIC 2025 work programme is adopted. Funding opportunities worth over €1.2 billion for strategic technologies and scaling up companies
EIC Pathfinder (grants up to €4 million): for multi-disciplinary research teams to undertake visionary research with the potential to lead to technology breakthroughs.
EIC Transition (grants up to €2.5 million): for turning research results into innovation opportunities, following up on results generated by EIC Pathfinder projects, European Research Council Proof of Concept projects and open for the first time to project results from Horizon collaborative projects from Pillar 2/ societal challenges.
EIC Accelerator: for start-ups and SMEs to develop and scale up innovations with the potential to create new markets or disrupt existing ones (grants below €2.5 million, investments from €0.5 to €15 million).
You can download the Work Programme using this LINK
Recording and presentations of the EIC Work Programme 2025 info days available using this LINK
2024
-
Dec9-10
Innovation Acta “Successful Proposal Writing & Funding Strategies Course”

On December 9th and 10th in Milan, we delivered an impactful course on Proposal Writing & Funding Strategies, designed to empower participants with the knowledge and tools needed to craft compelling proposals and navigate funding opportunities with confidence.
The sessions were interactive and insightful, featuring hands-on exercises that enabled participants to actively engage in discussions, sharing ideas, collaborating on real-world scenarios, thus immediately applying their learning.
As we look ahead, we are excited to offer more opportunities for professional growth and collaboration. Stay tuned for upcoming courses and events!
-
Dec5-6
ReSHAPE Final Meeting and Workshop on Next-Generation Treg Development
On December 5th and 6th, 2024, Berlin hosted the workshop on Next-Generation Treg Development, Product Characterization, and Biomarker Platform & Improvement of In Vivo Engraftment of Adoptively Transferred Tregs and final meeting of ReSHAPE. Organized by Innovation Acta, the event gathered around 70 participants.
The workshop featured insightful presentations on the project's research activities and achievements, delivered by the Project Coordinator and consortium partners. Renowned external experts in adoptive Treg therapy also contributed, sharing their cutting-edge findings and perspectives on this transformative field.
This gathering not only highlighted the significant progress made by ReSHAPE but also served as a vital opportunity to engage with the broader scientific community. By advancing knowledge critical to the future success of next-generation Treg therapies, the event reaffirmed its importance as a milestone in the evolution of adoptive Treg treatments.
-
Dec3-4
geneTIGA Second Annual Meeting
On December 3rd and 4th, 2024, the Second Annual Meeting of geneTIGA took place in Berlin, bringing together project participants and approximately 50 attendees. Organized by Innovation Acta, the two-day event featured comprehensive presentations and discussions that marked another milestone in the project’s progress.
Participants delved into the advancements achieved within each work package, showcasing the collective efforts of the consortium. The meeting also provided an essential platform for strategic discussions and planning, as partners outlined the roadmap for the next phases of the project.
The productive exchange of ideas and the shared commitment of all participants underscore the strong collaboration driving the project forward.

-
Dec2-4
TOLERATE 2nd Annual Meeting
From December 2nd to 4th, the TOLERATE Doctoral Candidates and project Partners came together for the 2nd Annual Meeting in Barcelona. Organized by AHT and INN-ACTA, the meeting included engaging training sessions on “Key Enabling Technologies” and a Biostatistics Workshop.
The attendees also had the unique opportunity to visit the Werfen Company, an associated partner in Barcelona. The Doctoral Candidates showcased their progress through presentations, collaborated with one another, and shared their personal experiences in the project, making it a valuable and enriching experience for all involved.

-
Nov12-13
B-specific 1st Annual Meeting
We recently (12th and 13th November 2024) participated in the 1st Annual Meeting of the B-specific (B-specific: B-cell related gene and protein markers with prognostic and therapeutic value for CVD) project ( https://www.bspecific.eu/ ), which was hosted by KU Leuven with support from Innovation Acta.
The meeting was a great opportunity to get an overview of the progress during the first year of the project and plan for the upcoming review meeting.

-
Oct4-5
Almost a year (1st December 2023) after starting the project Eco2Wine (Natural microbial interactions in winemaking-associated ecosystems as a tool to foster wine innovation) the consortium met in Stellenbosch, South Africa, on 4 & 5 October 2024.
Most of the beneficiaries, supervisors, co-supervisors, and doctoral candidates attended the meeting in person, while a few joined the meeting online.
The doctoral candidates' involvement in the event was vital as each presented their research plan and secondments, offering an excellent opportunity for discussion and planning collaborations. Speakers for each work package presented an overview of the activities and Innovation Acta provided an overview of administrative management tasks and presented activities with a focus on website development and maintenance.
-
Oct1-2
The fourth annual HDM-FUN meeting was held on October 1-2, 2024 nicely hosted by UZ Leuven. INNOVATION ACTA participated in the event, providing updates on project management, dissemination, communication activities, upcoming deadlines, and reporting requirements.
The meeting featured productive discussions and offered PhD students the opportunity to present their research. It concluded with a mini-symposium on the immunology of severe infections, including lectures from HDM-FUN principal investigators.

-
Oct1-2
We are pleased to announce the successful completion of our 2nd T-FITNESS Annual Meeting, which brought together all the partners of the Consortium coordinated by the Leibniz Institute for Immunotherapy. The event was organised by Innovation Acta and held in Sitges, Spain, on 1-2 October 2024.
The meeting fostered rich discussions and collaboration, significantly enhancing our shared knowledge. We were also pleased to welcome our new partner, Prof. Roman Jerala from the Kemijski inštitut (Slovenia), to the consortium. We extend our sincere gratitude to all attendees for their active participation, and a special thanks go to our advisory board members for their invaluable insights and guidance, which will play a crucial role in shaping our future initiatives. We look forward to continuing our collaboration and supporting this great consortium in advancing innovation!

-
Sept20
The fourth annual LiverScreen meeting was held on September 20, 2024, at Padova University Hospital. INNOVATION ACTA attended the meeting and shared updates on Project management, deadlines and reporting activities.
The annual meeting was organised in the context of the International Meeting on MASLD, organized by the University of Padova (Prof. Paolo Angeli and Prof. Robert Vettor) from September 20 to 21 2024 in Padova.
In conjunction with the MASLD Meeting, a LiverScreen Symposium was organized to showcase the project's contributions to the field. The symposium covered the following key topics:
- The impact of innovative technology (Laurent Castera, Paris)
- Screening of liver disease: is it cost effective? (Miquel Serra Burriel, Zurich)
- Best screening strategies in the general population (Maja Thiele, Odense)
- The LiverScreen project (Pere Gines, Barcelona)

-
Jul12
geneTIGA and T-FITNESS projects win fundings to include new partners from the Widening countries


GeneTiga and T-FITNESS projects, of which Innovation Acta is partner, have been recently granted by the EU Commission to include new partners and expertise from the widening countries. The two projects, supported by Innovation Acta, applied to the Hop On facility call with the aim to synergize with research groups from Genos (Croatia) and Kemijski Institut (Slovenia), respectively, and further boost the impact of the original projects. The EU introduced Widening to the framework research programme in 2014 under Horizon 2020, with the goal of helping countries lagging in research and innovation performance to close the gap with Europe’s top performers. This year Greece dominates the list of top performers for winning Horizon Europe funding among the 15 EU Widening countries, with six out of the top 10 places taken by the country’s institutions. While these measures are performing well in helping widening countries to strengthen their participation in transnational research and innovation processes, and promote networking and access to excellence, the imbalance between Widening countries and Europe’s top performers still persists and the EU’s next framework research programme FP10 is expected to continue funding projects aimed at bridging Europe’s research and innovation gap.
-
Jul12
The 2025 Work Programme of the European Research Council has been adopted!
-
Jul12
Switzerland will be eligible for ERC grants
Switzerland will be eligible for ERC grants, allowing Swiss legal entities to be treated as being “entities established in Associated Countries” for the Starting Grant, Consolidator Grant and Synergy Grant calls for proposals in the forthcoming ERC work programme 2025. See more details at this LINK
-
Jul12
Canada has now officially entered the Horizon Europe programme!
Canada has now officially entered the Horizon Europe programme! Canada has partnered with Pillar II of Horizon Europe, the largest collaborative part of the program with a budget of 52.4 billion euros, focused on four shared global challenges: climate, energy, digital economy, and health. Canadian researchers and organizations will have the opportunity to be directly funded by the program. See more details at this LINK
-
JUL12
ITALIAN CALL for PROPOSALS – LOMBARDY REGION
BANDO COLLABORA e INNOVA - REGIONE LOMBARDIA
Il Bando Collabora e Innova 2024 della Regione Lombardia, aperto a settembre 2024, mira a sostenere progetti complessi di ricerca, sviluppo e innovazione. I progetti, della durata di 27 mesi (prorogabili di massimo 6 mesi), devono essere realizzati in partenariato tra imprese (PMI, grandi imprese) e organismi di ricerca (OdR), incluse università e IRCCS. I partenariati devono comprendere almeno tre e massimo otto soggetti, con almeno una PMI e un OdR, e ogni impresa o OdR può partecipare a un unico progetto come capofila. Il bando copre il 60% delle spese ammissibili per piccole imprese, il 50% per medie imprese e il 40% per grandi imprese e OdR. I progetti devono avere un importo minimo di 3.500.000 euro ed essere realizzati in Lombardia.
Vedesi per ulteriori dettagli Collabora & Innova (regione.lombardia.it)
-
JUN6-7
Successful 1st Annual Meeting of the IMMUTOL project
We recently attended the 1st Annual Meeting of the IMMUTOL (Advanced Antigen-Specific Dendritic Cells Based Therapy to Re-establish Tolerance in Immune-Mediated Diseases) project. The meeting took place on the 6th and 7th of June 2024 and had over 30 participants. It was hosted and organized by the team of Assistant Professor Jordi Martorell López at the Department of Chemical Engineering and Materials Science in Barcelona, Spain, with support from Innovation Acta. The event provided a comprehensive update on the project's progress. Speakers for each work package shared updates, and participants engaged in a roundtable discussion led by relevant partners. During the meeting, we provided an overview of administrative management tasks and presented the activities focused on communication, dissemination, exploitation, and citizen engagement together with ADEMTO.

Photo of participants

First day of the meeting
-
MAY21-24
TOLERATE 2nd Progress Meeting and Mid-Term Meeting
Last week, from the 21st to the 24th of May, the TOLERATE consortium convened in Antwerp, Belgium, for the 2nd Progress Meeting and Mid-Term Meeting. The 8 TOLERATE Doctoral Candidates and their supervisors attended and greatly contributed to the success of the meeting organised by anicells and INN-ACTA. We were privileged to have the EU Project Officer, Giuliana Donini, in attendance for the Mid-term meeting.
Witnessing everyone's dedication and enthusiasm as we reviewed the latest research progress from our students was truly inspiring. Together with the presentations from DCs, the meeting also included the Transferable skill courses on “Managing innovation, knowledge and data” and “Clinical development of novel therapies of TTP and the impact and role of in vitro diagnostics on patient selection and trail enrichment” and a visit at anicells facilities in Niel.
-
MAY23
Enhancing Expertise: A Recap of Our Recent Educational Events
We are thrilled to share a summary of our recent educational endeavours, where we delved deep into the realms of project management and strategic planning. At Inn-Acta we are , committed to fostering knowledge exchange, catalyze growth and innovation thus empowering professionals to excel in their fields. Here's a glimpse into the events we've organized between March and May 2024:
Courses on Research Integrity and Science Communication
In February 2024, we organized an immersive course in Amsterdam, designed to equip PhDs of a Horizon Europe Doctoral Network with skills in research integrity and communication in science. Through interactive sessions and hands-on workshops, participants gained invaluable insights into EU principles for Integrity and Responsible Research and into communication and Dissemination in European projects, including Science Communication using video platforms. Professional experts led the discussions, including also our team members Gabriella Dessole and Laura Maccari ensuring a comprehensive understanding of the principles tailored to the needs of responsible research and modern communication tools.
Open science, Research Infrastructure, and project management
As part of our dedication to continuous learning, we assumed the role of organizers for a multifaceted event featuring a lineup of esteemed speakers. Held in Rome in May 2024 this event brought together thought expert speakers to explore strategies in Open science, Research Infrastructure, and project management and beyond. Attendees had the opportunity to engage with experts, exchange ideas, and network with like-minded professionals, fostering a vibrant community of learning and collaboration.
Project Management and Grants Management Course:
Our team members Natascia Cannella and Nicoletta Corti had the privilege of contributing as speakers at formative course in Brescia, Italy for IZSLER - Experimental Zooprophylactic Institute of Lombardy and Emilia Romagna “Bruno Ubertini” - focused on Project and Grants Management key concepts and methodologies on May 23rd . Leveraging our expertise and experience, we delivered interactive presentations and held lively discussions aimed at empowering participants for their future projects’ setup and monitoring with practical tools.
Through these events, we reaffirm our commitment to providing tailored educational opportunities and fostering a culture of continuous learning and development. Stay tuned for future initiatives as we continue to empower individuals and organizations to thrive in an ever-evolving landscape.
For more information about our educational services and upcoming events, please visit the page https://www.innovationacta.eu/training-courses or contact us at info@innovationacta.eu
-
MAY21-22
TOP-GUT First Networking Event
We attended the Kick-off Meeting (KoM) with the Doctoral Candidates (DCs) of the MSCA-DN project TOP-GUT (Training for Organoids modelling Physiology and Pathology in the human gastrointestinal tract). The meeting, held between May 21st and 22nd, was organized by the team of Prof. Sina Bartfeld, coordinator of TOP-GUT at the Technische Universität Berlin, with support from Innovation Acta.
The KoM was a great opportunity to gain an overview of the scientific background of the project, thanks to the excellent keynote speakers, delve into the details of the individual DC projects and interact with the bright, energetic, and dedicated DCs and their supervisors, who are leaders in their specific fields.
Innovation Acta also provided an overview of the project management and communication & dissemination related activities and obligations.
We wish all the best for all DCs in their 3-year scientific adventure and career development!
-
APR29-30
2nd Annual General Assembly of Innovative Health Initiative (IHI) GUIDE.MRD Consortium
The Innovative Health Initiative (IHI) GUIDE.MRD Consortium partners met for the 2nd Annual General Assembly hosted by the coordinating institution at the Universitätsklinikum Hamburg Eppendorf (UKE), from 29-30 April 2024 and organized with the support of Innovation Acta S.r.l. (INN-ACTA).
After a warm welcome to the beautiful city of Hamburg by the Project Coordinator Klaus Pantel (UKE) and the Project Leader Bristol Myers Squibb (BMS) represented by Luigi Ravagnan, the progress on all work packages was presented by the work package (WP) leads and co-leads.
Moreover, a fruitful plenum discussion with all participants on current challenges and mitigation strategies as well as the next steps within the consortium was held.
Over 30 participants from the 25 partners engaged in very productive discussions on the results reached by the consortium in the first 12 months.
We would like to thank all partners involved!

-
APR16-17
Successful DoC Kick-Off Meeting Marks the Beginning of exTra Doctoral Network Project!
We have attended the DoC kick-off meeting of extra, a doctoral network project focused on Extracorporeal Photopheresis (ECP) in solid organ transplantation. The meeting, organized by Innovation Acta, was held in Regensburg on 16-17 April and brought together project partners for 2 days filled with insightful discussions and promising presentations from 11 talented doctoral candidates.
Over the next 36 months, these doctoral candidates will serve as the driving force behind exTra project, leveraging their expertise to generate groundbreaking data and insights. Their work will be instrumental in advancing understanding of ECP and paving the way for its translation into clinical practice.

-
APR12
Doctoral Networks Call 2023 - Evaluation Results
The European Commission has completed the evaluation of the 2023 MSCA Doctoral Networks (DNs) call and published the evaluation results on 12 April 2024.
Of the 1,066 submitted proposals, 138 were invited for grant preparation.
It is expected that the first grant agreements will be signed by July 28th 2024.
Information on the selected projects will be published on CORDIS after that date.
The projects’ starting date will be between 01/09/2024 to 01/03/2025.
-
APR1
EIC Women Leadership Programme: Open Call – for female researchers and aspiring female leaders
The European Innovation Council (EIC) is pleased to announce the launch of the 5th cohort of the EIC Women Leadership Programme (EIC WLP). Building on the success from the previous editions, this cohort, running from April - June 2024, will offer EIC female researchers and aspiring female leaders the tools and support to enhance their leadership skills.
more info

-
MAR20
Next Event - Interreg Europe
Call open from 20 March to 7 June 2024
Program Manual here
Sign-up for the online edition of Europe, let's cooperate interregional cooperation forum, which will take place on 20th March 2024 live from Antwerp. Registration is open!

-
MAR29
Switzerland Advances Toward Eligibility for Horizon Europe Participation
The European Council has recently adopted a negotiating mandate with Switzerland. Upon the formal commencement of talks, transitional measures will be implemented, allowing applications to the European Research Council (ERC) this year.
EU-Switzerland: Council adopts mandate for negotiations on future relationship - Consilium (europa.eu)
These transitional measures are anticipated to extend to all Horizon Europe calls throughout the program year 2025. However, their activation is contingent upon mutual agreement between chief negotiators from both sides on an association deal. It is conceivable that this deal will be endorsed by EU and Swiss authorities before the conclusion of 2025.
Notably, Swiss researchers will not receive any funding until the association agreement is officially ratified. The first ERC call potentially accessible under these arrangements is the 2024 advanced grants, scheduled to commence on 29th May.
-
MAR21-22
LIVERAIM Kick-off Meeting
The LIVERAIM project kicked off on March 21st and 22nd with representatives from all of our 27 partners, involving researchers, SMEs, biotechnology companies and the pharmaceutical industry to start a public-private collaboration of 25 million € invested by the Innovative Health Initiative and industry for the development of a biomarker-based platform for early diagnosis of chronic liver disease to enable personalised therapy.
The two-day meeting was organised by Nicola van Berckel from FRCB-IDIBAPS with the support of Innovation Acta and gently hosted by Prof. Pere Ginès at the Faculty of Medicine and Health Sciences of the University of Barcelona (Spain). The first day was organised in the form of an open event to discuss about the global burden of chronic liver diseases highlighting the gaps, the challenges, and successes in tackling this issue. In the second day, the meeting gathered more deeply on LIVERAIM project by presenting workplan and objectives and scheduling the upcoming activities. INNOVATION ACTA attended the meeting and shared updates on Project management, deadlines and reporting activities.
Representatives of IHI Research Programme, industry, State Government of Catalunia, and high representatives of FRCB-IDIBAPS, Hospital Clinic, and University of Barcelona were invited to the meeting to give high relevance of the project at different level and stakeholders.

First day speakers 
LIVERAIM consortium members
-
MAR12
GLYCO-N Kick-off Meeting
The GLYCO-N partners met in Naples on 12th March 2024 for the Kick off meeting to get to know each other, and plan future project activities and the recruitment of Doctoral Candidates. The event was hosted by the Department of Chemical Sciences, University of Napoli Federico II and nicely organised by Prof Antonio Molinaro with the support of Innovation Acta. Innovation Acta attended the meeting and shared updates on Project management, recruitment process and Project website.


-
MAR1
LIVERAIM - Starting from March 1st 2024, European researchers will conduct the largest trial ever in liver disease
Liver diseases account for 300,000 deaths in Europe each year. With support from the Innovative Health Initiative, a collaboration of researchers, biotechnology companies and the pharmaceutical industry aim to improve the lives and health of people with hidden liver disease by conducting the largest study ever on early detection of liver damage.
Cirrhosis and liver cancer pose a significant burden on healthcare systems, impacting quality of life, work productivity, and necessitating specialized medical care. The key risk factors for both cirrhosis and cancer include obesity, type 2 diabetes, and increased alcohol consumption—trends that are on the rise across Europe and globally. Unfortunately, these diseases often progress silently until severe symptoms and complications arise. While liver transplantation is an effective treatment, it is not universally accessible.
However, if liver disease is detected early, during the fibrosis stage, severe outcomes can be reversed and prevented. Enter the LIVERAIM project: a collaboration of renowned clinical centres and industrial partners, including SMEs, with expertise in Liver Disease. Their goal is to design and validate a screening platform with biomarkers for population-wide use in Europe. The objective? Early identification of liver disease and personalized therapeutic interventions.Here’s how LIVERAIM works:
- Biomarker Testing: Existing biomarkers will be evaluated for their accuracy in predicting fibrosis. This analysis will involve 30,000 plasma samples from previously H2020 EU-funded cohorts.
- AI-Powered Screening Platform: Using Artificial Intelligence (AI), the partners will develop a screening platform for personalized early diagnosis of liver fibrosis.
- Validation: The platform will be rigorously validated in a randomized controlled trial (RCT) involving 100,000 subjects from six representative EU countries.
- Tailored Interventions: The platform will link to personalized therapeutic interventions, aiming to halt fibrosis progression.
By enabling early diagnosis and personalized interventions, LIVERAIM aims to reduce morbidity, mortality, and the associated economic and health inequities related to liver disease.
-
FEB27
PROTO First Annual meeting
The PROTO partners met on Salzburg on 27th February 2024 for the First project Annual meeting to share the advancements achieved in the first year and discuss on the challenges ahead. As a partner delivering project management and communication activities INNOVATION ACTA attended the meeting and shared updates on Project management, reporting activities, progresses on the Communication activities and Project website. At the heart of PROTO collaborative effort is the mission to address inflammation, the pivotal factor in the progression of Osteoarthritis. The consortium comprises leaders in various fields such as omics, cellular immunology, molecular biomarkers, physiology, biomechanics, and stem cell biology. Together, PROTO is actively engaged in groundbreaking clinical studies, notably with advanced therapy medicinal products. A significant milestone on horizon is the initiation of a first-in-human phase I/IIa study. This study focuses on patients with moderate knee osteoarthritis and investigates the promising anti-inflammatory PLX-PAD cell therapy. Simultaneously, PROTO is implementing an innovative, high-level personalized sensor- and app-based training intervention for patients in the pre-stage of Osteoarthritis. This multifaceted approach underscores partners’ commitment to addressing the complex facets of this debilitating condition. Integral to PROTO project are cutting-edge ancillary studies aimed at defining immune-mediated mechanisms both in and ex vivo. Additionally, the initiative seeks to identify a spectrum of biomarkers, including immunologic, metabolic, genetic, and MRI-based morphologic markers. This comprehensive approach enables precise patient stratification, a crucial step towards more effective and personalized interventions.

-
FEB26
Innovation Acta was honored to contribute in the INFO DAY HORIZON EUROPE – EU PARTNERSHIPS 2024
The event took place on February 26, 2024, organized by APRE in collaboration with MUR and in partnership with Confindustria Dispositivi Medici, Confindustria, Farmindustria, and Federchimica Assobiotec. Our CEO Natascia Cannella presented two succesfully funded IHI projects (GuideMRD and LiverAIM). It was an informative day dedicated to the new calls 6 and 7 of the IHI partnership, aimed at promoting awareness and information among companies, hospitals, and research centers.
More info
-
JAN25-26
NECESSITY Fifth General Assembly
The NECESSITY project held its annual General Assembly with representatives from all of our 25 partners, including both from industry and academia. We were also honored to have the indispensable Patient Advisory Group (PAG) representatives among us. The two-day meeting was hosted by our industry partner, GSK in Stevenage (London, UK). The main objectives of the two days were to facilitate discussions within the NECESSITY Project Consortium on key advances and innovative approaches in the fields of immunology and rheumatology. More importantly, the meeting aimed to address any challenges that arose and to propose solutions for the smooth progression of the project. Exciting new methodologies and results were presented for the stratification of patients based on tissue and blood biomarkers. Additionally, we delved into an emerging approach in computational biology to evaluate the pathophysiology of Sjogren’s disease. Furthermore, we witnessed the first examples of the scientific community using STAR.

-
JAN12
THRIVE Kick off meeting
The European Union’s THRIVE project (Tumor host interactions in liver cancer for childhood and adults) held its kick-off meeting on Friday, 12 January 2024. Thirty-five people attended the meeting, which took place in the Esteve Auditorium of the Esther Koplowitz Centre, where IDIBAPS’ headquarters are located.

Members and participants of the kick-off meeting of the THRIVE project
More information about the project here
2023
-
NOV20-22
1st TOLERATE Annual Meeting – 20-22 November 2023, Amsterdam

The 1st Annual Meeting for the TOLERATE project has been held in Amsterdam from the 20th to 22nd of November. The event has been organised by the project partner Sanquin with the support of Innovation Acta. The 3-days meeting included the progress meeting with presentations from all the fellows, and three different training courses on scientific and transferable skills.
-
NOV14-15
geneTIGA Annual Meeting in Enchanting Rome on 14th -15th November

On 14 and 15 November 2023 INN-ACTA organized the First Annual meeting of geneTIGA project in Italy.
geneTIGA is a Research and Innovation Action funded under Horizon Europe that aims to develop a safe and efficient cell therapy based on genome-edited T cells with redirected specificity to sustainably combat IgA nephropathy (IgAN). geneTIGA specific cell therapy approach is also suitable for other diseases with selective B-cell pathogenesis, such as IgA myeloma and rheumatoid arthritis, but as a blueprint also for diseases of other Ig classes.
We had the pleasure of gathering all consortium members from Germany, Spain, Norway, Denmark, UK and Switzerland, in the breathtaking city of Rome for a very productive and inspiring meeting. The meeting provided a valuable opportunity for us to discuss about the work accomplished and progresses achieved in the past year and about the new challenges ahead. The exchange of ideas was lively, with each member contributing their unique perspectives and expertise to the conversation.
Rome, with its rich history and cultural significance, provided the perfect backdrop for fostering a sense of unity among consortium members.
We look forward to leveraging the insights gained during this meeting to propel our project forward. -
OCT22-23
GUIDE.MRD First face-to-face meeting on 22nd and 23rd of October in Madrid

The GUIDE.MRD consortium partners met for the first face-to-face annual meeting hosted by Dr. Núria Malats at CNIO - Spanish National Cancer Research Centre, in Madrid, Spain, from 22-23 October 2023 and organized with the support of Innovation Acta S.r.l.
During the first day first day the monthly Steering Committee meeting took place in presence, chaired by the Academic Coordinator, Prof. Klaus Pantel, Universitätsklinikum Hamburg Eppendorf and the Project Leader, Bristol Myers Squibb, followed by a networking evening hosted by CNIO with fruitful discussions on the GUIDE.MRD project and most recent data from ESMO 2023.
The second day was opened by welcoming remarks from the Academic Coordinator and a presentation on the hosting partner CNIO. Thereafter, the Work Package leaders Ellen Heitzer (Medical University Graz, Austria, WP2) and Claus Lindbjerg Andersen (Aarhus University Hospital, Denmark, WP3) as well as clinical study leaders Matthias Löhr (Karolinska Institute, Sweden, WP3) and Jeroen Hiltermann (University Medical Center Groningen, The Netherlands, WP3) presented the most recent data and progress of the project.
Over 40 participants from the 22 partners engaged in very productive discussions on the results reached by the consortium in the first 6 months as well as some of the main challenges and opportunities. It was also noted how input from patients has been incorporated since this early phase of the project through our partners The Synergist, Lung Cancer Europe, Digestive Cancers Europe and the project's Patients Advisory Board, especially in relation to the first three clinical trials. -
OCT23
The B-specific Kick-Off meeting, 23rd October 2023 in Leiden

The B-specific consortium led by Amanda Foks (Leiden Academic Centre for Drug Research, Leiden University, The Netherlands) successfully kicked-off the project B-specific (B-cell related gene and protein markers with prognostic and therapeutic value for CVD). The event was held on the 23rd of October 2023 at the Faculty Club (Academy Building) in Leiden (The Netherlands). Thanks to European Innovation Council (EIC) (Grant Nr. 101115159) for supporting the research to discover new biomarkers and therapeutic targets for atherosclerosis.
-
OCT01
B-specific project starts on the 1st of October 2023

We are glad to announce that the B-specific project, funded by the European Innovation Council under the 2022 Pathfinder Challenge call will officially start on the 1st of October 2023. During the next four years, the international consortium coordinated by Leiden University (The Netherlands) will focus on the discovery of novel biomarkers of cardiovascular disease to accelerate the impact of these in clinical practice. Innovation Acta, as part of the Consortium, will assist the administrative bodies of the Partners’ institutions and will provide support to communication activities. The Kick-off meeting will be held on the 23rd of October 2023 in Leiden (The Netherlands).
-
SEP18-19
T-FITNESS meeting in Capri

On the 18th and 19th of September, the T-FITNESS consortium met in the beautiful island of Capri for the first annual meeting. This EIC Pathfinder grant aims to explore bold ideas for radically new technologies. In particular, T-FITNESS aims to revolutionize current T cell immunotherapies by developing an innovative platform to overcome T cell exhaustion. During the meeting, the partners discussed the remarkable progress made within just one year of the project's inception and exchanged valuable insights regarding the challenges ahead.
-
SEP11
It’s official: UK to associate to Horizon Europe
After multiple false dawns, London and Brussels have finally agreed a deal to allow the UK to rejoin. The UK will also join the Copernicus space programme but is out of Euratom and the ITER fusion project. UK will be able to fully participate from 1 January 2024 in the 2024 work programme – a list of the projects Horizon Europe will fund that year - and onward, including 2024 calls that open this year. For calls under the 2023 work programme, however, UK researchers will still have to use UK backup guarantee scheme to fund their participation in any Horizon projects. The agreement is only until the end of Horizon Europe in 2027.
Full text and additional articles here:
It’s official: UK to associate to Horizon Europe | Science|Business (sciencebusiness.net)
How the UK and EU did a deal over Horizon Europe | Science|Business (sciencebusiness.net)
Science world welcomes UK back to Horizon as government celebrates ‘bespoke deal’ | Science|Business (sciencebusiness.net) -
SEP01
01 September 2023 exTra Project officially started!

exTra is a Doctoral Network funded under Horizon Europe Programme. exTra Consortium is a research network of leading European scientists from academia and industry, experts in clinical transplantation, immunology, pharmaceutical development and medical device manufacture to address key questions on extracorporeal photopheresis (ECP) through a coordinated, interdisciplinary effort. exTra proposes 11 independent doctoral research projects with the ambition of providing its trainees with a comprehensive understanding of basic and translational immunology, especially relating to development and licensing of new immunotherapies. Through its research and training activities, the exTra project will contribute to scientific advancement and innovation in Europe, ultimately leading to societal and economic benefits stemming from clinical innovations in transplant immunotherapy and beyond. Innovation-Acta is Associated Partner of the Network for management, training and communication activities.
-
MAY-JUN30-1
INsTRuCT final meeting on 31st May and 1st June 2023 in Regensburg
INsTRuCT Network members from 13 beneficiaries, the 15ESRs, the associated industrial partners Sanofi, AstaZeneca, UCB Biopharma and InnovationActa, met in Regensburg for the final meeting. The meeting included outstanding presentations from all 15ESRs, now at the third year of their research work, who reported about their individual projects and achievements in the in the myeloid regulatory cell (MRC)-based therapy research. A Networking session for ESR and commercial partners was part of the programme. INsTRuCT is funded under Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860003. The project combines excellence in basic, preclinical and translation research with an outstanding programme of scientific education and practical training for early stage researchers, who are driving critical advancement in the field.
-
MAY24-25
IMMUTOL Kick off meeting on 24th and 25th of May 2023 in Milan

The IMMUTOL Consortium, led by Silvia Gregori (San Raffaele Telethon Institute for Gene Therapy– SR-Tiget) successfully kicked-off the project "Advanced Antigen-Specific Dendritic Cell-Based Therapy to Re-establish Tolerance in Immune-Mediated Diseases". The hybrid event was held on 24th and 25th of May at Fondazione Telethon, Milano (Italy). Innovation Acta organized and joined the meeting. Thanks to EU Horizon Europe (Grant Agreement Nr.: 101080562) for supporting fast-tracking the development and validation of an Advanced Therapy Medicinal Product based on engineered dendritic cells to treat patients with immune-mediated diseases with an unmet medical need, in particular multiple sclerosis.
-
MAY1
GUIDE.MRD project starts on 1st May 2023

On May 1st 2023 just started GUIDE.MRD, a five-year Innovative Health Initiative project. GUIDE.MRD is a consortium of leading academics, technology companies, pharmaceutical companies, patient organizations, and experts in multi-stakeholder engagements. Innovation Acta, as part of the Consortium, will provide support to the administrative management of the project, and to communication activities. GUIDE.MRD will tackle the critical questions by developing reference standards for circulating tumor DNA (ctDNA) diagnostics, clinically validate promising ctDNA diagnostics and develop data to guide the use of multi-modal therapies with a noninvasive diagnostic test. With robust engagement with regulatory authorities, payers and importantly patients themselves, we will develop recommendations and guidelines based on objective data to use ctDNA diagnostics to guide multimodal therapy selection to improve patient outcomes. GUIDE.MRD Kick off meeting will be held online on 29th – 30th June 2023
-
MAY1
IMMUTOL project starts on 1st May 2023

We are thrilled to announce that 1st of May 2023 is the official start of IMMUTOL. For the next four years, in the frame of this Research and Innovation Action, the main goal will be to fast-track the development and validation of an advanced therapeutic medicinal product, aimed to treat patients with immune-mediated diseases. IMMUTOL will develop a more potent and durable treatment for autoimmune diseases with an unmet medical need, in particular Multiple Sclerosis.
The consortium gathers experts and institutions from different sectors, from research institutions and academia, patients’ association to hospitals and corporate SME. Innovation Acta, as part of the Consortium, will provide support to the administrative management of the project, and to communication activities.
The Kick-off meeting will be held on 24th and 25th of May 2023 in Milano (Italy). -
APR19
Doctoral Networks Call 2022 - Innovation Acta participation
We are pleased to be Partner in 4 Doctoral Networks projects selected in the MSCA Call 2022, reaching 100% of success rate in this call.
In these DNs, we provide professional assistance with Project Management, communication activities and training programmes with our transferable skills courses. -
MAR16
Doctoral Networks Call 2022 - Evaluation Results
The European Commission has completed the evaluation of the 2022 MSCA Doctoral Networks (DNs) call and published the evaluation results on 16 March 2023.
Of the 946 submitted proposals, 149 were invited for grant preparation, resulting in an overall success rate of 15.8% for the 2022 call. The success rate in the 2022 call is 2.4 percentage points higher than in 2021 (13.4%).
It is expected that the first grant agreements will be signed by July 15th 2023.
Information on the selected projects will be published on CORDIS after that date.
The projects’ starting date will be between 01/09/2023 to 01/03/2024 -
MAR1
PROTO Kick-off meeting 28th February-1st March 2023 in Berlin

We successfully kicked-off our PROTO consortium during these exciting two last days! Thanks to EU Horizon Europe (Grant Nr. 101095635) for supporting our endeavour to prevent, early diagnose and treat osteoarthritis.
-
FEB13
Extension to support UK Horizon Europe applicants
UK Government ensures that eligible, successful UK applicants will continue to be guaranteed funding. The guarantee will be in place to cover all Horizon Europe calls that close on or before 31st of March 2023. Eligible, successful applicants to Horizon Europe will receive from the UK Research and Innovation (UKRI) the full value of their funding at their UK host institution for the lifetime of their grant. View more
To be eligible the applicant must: (i) be based in the UK; (ii) have been successful at applying for a Horizon Europe grant with final submission deadlines on or before 31st of March 2023 and (iii) have been included on the initial grant proposal as a ‘beneficiary‘ with an assigned budget. In parallel, the UK government continues to push for Association to EU programs.
-
JAN1
PROTO project officially started!
January 1st marks the official start of PROTO, a five -year international project coordinated by Charité - Universitätsmedizin Berlin, Germany. The Kick-off meeting will be held in Berlin on 28 February-1st March 2023. PROTO will develop novel interventional strategies aimed at tackling the development and progression of osteoarthritis. The PROTO consortium gathers experts and institutions from different sectors, from academia to SMEs and patient organizations. They all focus on distinct and complementary steps in the clinical research on musculoskeletal diseases, from ATMP manufacturing and characterization, their application in the clinic, implementing biomarker analysis, metabolomics and genetic profiling and patient input and information. Innovation Acta is part of this great team and ambitious project’s goal.
2022
-
DEC21
Innovation Acta wishes you a relaxed Christmas time, Nice Holiday Season and a Happy, Healthy and Successful 2023!

-
DEC20
New Health Cluster Work Programme
The new Work Programme 2023-2024 of the Health Cluster has been published. In our website you can find a selection of calls, while the whole Health Work Programme is available at the link below
-
DEC20
New IHI Work Programme
The 3rd IHI call is now open with a deadline on March 15th, 2023. Topics are:
- Topic 1: Screening platform and biomarkers for prediction and prevention of diseases of unmet public health need
- Topic 2: Patient-generated evidence to improve outcomes, support decision making, and accelerate innovation
- Topic 3: Combining hospital interventional approaches to improve patient outcomes and increase hospital efficiency
- Topic 4: Strengthening the European translational research ecosystem for advanced therapy medicinal products (ATMPs) for rare diseases
- Topic 5: Digital health technologies for the prevention and personalised management of mental disorders and their long-term health consequences
-
DEC20
New EIC Work Programme
The new EIC Work Programme 2023 has been published. Main calls in 2023 are summarised below
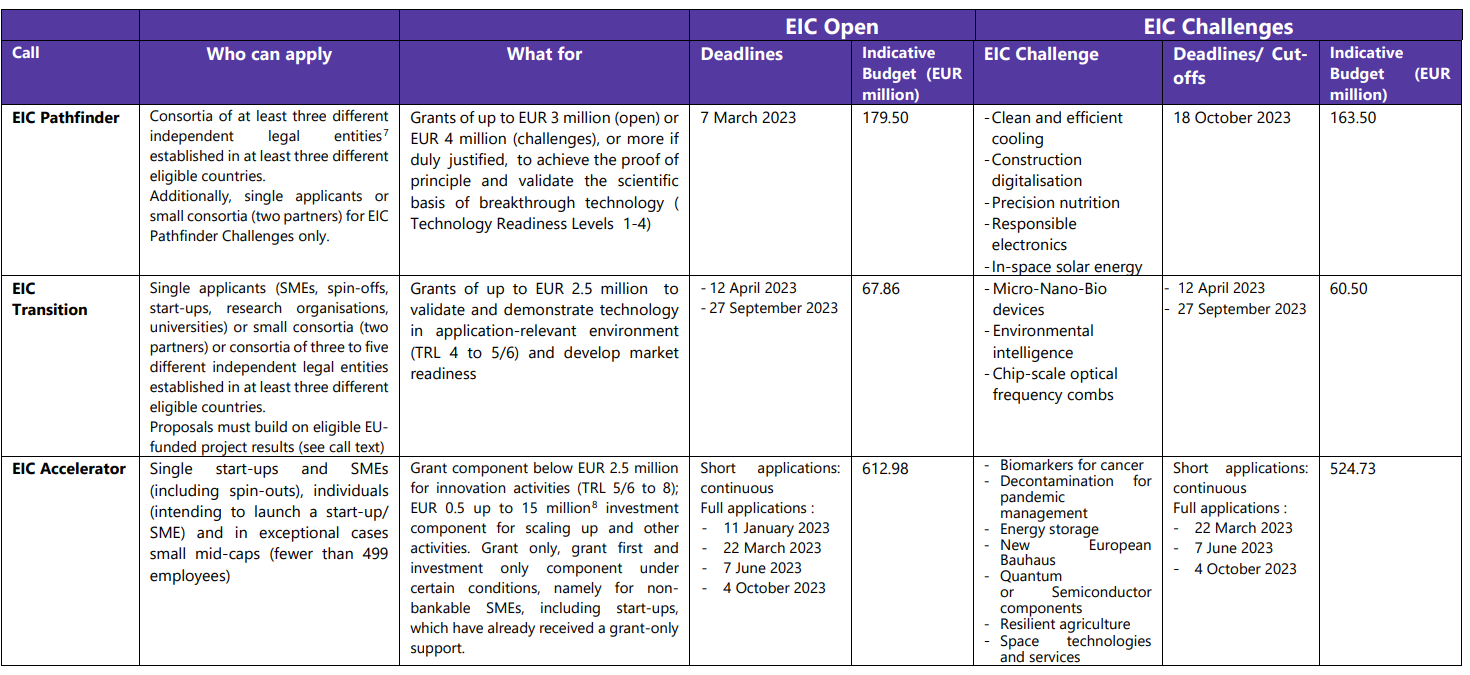
-
DEC20
New Mission Work Programme
The new Mission Work Programme 2023-2024 has been published. In our website you can find a selection of calls, while the whole Mission workprogramme is available at the link below
-
DEC20
New MSCA Work Programme
-
OCT01
TOLERATE starts today!

October 1st marks the official start of TOLERATE, a Marie Skłodowska-Curie Doctoral Network (MSCA-DN) funded by the European Union Horizon Europe Programme. TOLERATE aims at training 8 Doctoral Candidates to acquire the skills to develop different innovative strategies to treat autoimmune diseases, to identify the most promising strategies or a combination of strategies, to setup clinical trials and to develop a roadmap to bring a novel therapeutic agent to the market. As Associated Partner, Innovation Acta will provide support to the administrative management of the project, and to communication activities.
-
SEP01
T-FITNESS project official start

September 1st marks the official start of T-FITNESS, a four-year international project coordinated by the Leibniz Institute for Immunotherapy (LIT), Regensburg, Germany. T-FITNESS is one of 39 projects -out of 403 proposals- selected for funding by the European Innovation Council in the 2021 Pathfinder Challenge call. Innovation Acta will provide support to this great team and ambitious project’s goal aiming to revolutionize current T cell immunotherapies.
-
JUL22
The European Commission has recently launched a pilot evaluation process within the EIC Pathfinder programme under the so-called "Rebuttal Phase"
-
FEB16
StairwAI 1st Open Call

Artificial intelligence: the first call of the StairwAI project is now open.
StairwAI, is a European Union-funded project which aims to facilitate the engagement of low-tech companies in the AI4EU platform (a one-stop-shop for anyone looking for Artificial Intelligence (AI) knowledge, technology, tools, services, and experts) and the implementation of AI.
The project has now launched its first open call or funding opportunity for low-tech small and medium-sized companies (SMEs) that do not have immediate access or knowledge on AI techniques to improve their products, services, or value chains with the support of AI Experts and High Computing Cloud Providers.
-
FEB15
NextGenerationEU

NextGenerationEU: European Commission raises 5 billion in additional funds for recovery
With the first bond issue by syndication in 2022, the European Commission raised today an additional €5 billion in NextGenerationEU funds on behalf of the EU. Maturing on 6 July 2051, the 30-year bond, executed as an increase in an existing EU bond, brings the total funding raised under the programme to €78.5 billion. The successful placement of the Commission will help to boost Europe’s recovery from the COVID-19 pandemic.
-
FEB15
Ideas Powered for Business

Intellectual property: launch of the "Ideas Powered for Business" website, the new information hub for European SMEs
The European Intellectual Property Office (EUIPO) has created a centralised information hub for businesses interested in intellectual property aspects. With the new “Ideas Powered for Business” website, the EUIPO is intensifying efforts to help entrepreneurs, start-ups, and small to medium-sized enterprises (SMEs) to alleviate some of the challenges they face in relation to intellectual property (IP).
The platform offers information geared towards supporting SMEs and other stakeholders to develop their business strategy and accelerate business growth with a clear focus on IP and IP rights, such as a training area, a database of IP services, an events section, and other resources.
-
FEB09
EIC Work Programme 2022

The Commission adopted today the 2022 work programme of the European Innovation Council. It opens funding opportunities worth over €1.7 billion in 2022 for breakthrough innovators to scale up and create new markets, for example in quantum computing, new generation batteries and gene therapy. Launched in March 2021 as a major novelty of the Horizon Europe programme, the European Innovation Council has a total budget of over €10 billion between 2021 and 2027.
-
FEB09
Linee Guida per le iniziative PNRR

Sono state pubblicate le Linee Guida del Ministero della Salute e MUR per i prossimi bandi del Piano complementare al Piano Nazionale di Ripresa e Resilienza per le Iniziative di ricerca per tecnologie e percorsi innovativi in ambito sanitario e assistenziale e per l’Ecosistema innovativo della salute.
-
JAN18
Commission selects first EIC Transition projects to take breakthrough technologies from the lab into the real world

The European Innovation Council (EIC) has selected 42 projects following a first ever EIC Transition calls for proposals. Successful proposals, selected among 292 submitted, will receive altogether €99 million of EU funding.
-
JAN18
ERC-funded Research wins most of new eu innovation grants

25 out of 42 recipients of the European Innovation Council’s new “Transition” funding originated from research funded by the ERC. The new grants will help take breakthrough technologies from the lab into the real world.
-
JAN14
Open Mission Calls

EU Missions new work program of Horizon Europe: 122.38 million euros are allocated to the first calls of the Mission "Adaptation to Climate Change", which opened on 11 January 2022. The Mission aims to support regions and communities in their adaptation to climate change, including the development of risk assessments, pathways and demonstrators of large-scale innovative systemic solutions that are cost-effective.
Mission: Adaptation to climate change (calls detail)
EU Missions new work program of Horizon Europe: 119.37 million euros are allocated to the first calls of the Mission "Climate-Neutral and Smart Cities", which opened on 11 January 2022. Other topics will open on April 28, 2022. The Mission intends to invest in new urban planning and design for sustainable, resilient and climate neutral cities, to unlock the innovation potential of public transport as the backbone of urban mobility and to create districts of positive clean energy.
Mission: Climate neutral and smart cities (call details)
EU Missions new work program of Horizon Europe: 62 million euros are allocated to the first calls for the Mission "Soil health and food", opened on 22 December 2021. Funded projects will support the creation of living labs on soil health and the validation and definition of soil health indicators, as well as the creation of a new generation of soil consultants to guide landowners and land managers in implementing sustainable practices, as well as to guide authorities in decision making for a positive impact on soil health.
-
JAN14
EU Missions Cancer

EU Missions new work program of Horizon Europe: 125.65 million euros are allocated to the first calls of the "Cancer" Mission, open from 22 December 2021. The Mission intends to develop new methods and technologies in cancer screening and early diagnosis, establish measures for the quality of life, as well as understand the impact of risk factors and health determinants on cancer development and progression.
-
JAN12
Horizon Europe: Tackling diseases 2022

New call: Tackling diseases (Single Stage - 2022). This call aims at supporting activities that are enabling or contributing to one or several expected impacts of destination 3 “Tackling diseases and reducing disease burden”.
-
JAN12
Partnerships in Health 2022

New call: European partnership fostering a European Research Area (ERA) for health research. The call is part of Destination 2 - Living and working in a health-promoting environment which aims to promote sustainable and health-friendly living and working environments thanks to a better understanding of environmental, occupational, social and economic risk factors.
-
JAN10
JPIAMR 14th Call

JPIAMR is launching an international call for projects under the umbrella of the JPIAMR and within the framework of the ERA-NET JPIAMR-ACTION. The call Disrupting drug resistance using innovative design involves 27 funding organisations from 18 countries. The total estimated call budget is close to 19 million Euro.
-
JAN10
Open calls “LIFE”

New call: LIFE-2021-Preparatory Projects as part of the Programme for the Environment and Climate Action (LIFE) Call for proposals. The call is launched in accordance with the 2021-2024 Multiannual Work Programme2 and will be managed by the European Climate, Infrastructure and Environment Executive Agency (CINEA) (‘Agency’).
-
JAN10
PNRR Italian calls

Nuovo bando per gli ecosistemi dell’innovazione territoriali
La misura prevede un investimento complessivo di 1,3 miliardi di euro.
Nuovi bandi bandi per le infrastrutture di ricerca e per le infrastrutture tecnologiche di innovazione
La misura prevede un investimento complessivo di 1,58 miliardi di euro.